Biomedical engineering is the application of engineering principles and techniques to the medical field. This field seeks to close the gap between engineering and medicine. It combines the design and problem solving skills of engineering with medical and biological sciences to improve healthcare diagnosis and treatment.
Biomedical engineering has only recently emerged as its own discipline, compared to many other engineering fields; such an evolution is common as a new field transitions from being an interdisciplinary specialization among already-established fields, to being considered a field in itself. Much of the work in biomedical engineering consists of research and development, spanning a broad array of subfields (see below). Prominent biomedical engineering applications include the development of biocompatible prostheses, various diagnostic and therapeutic medical devices ranging from clinical equipment to micro-implants, common imaging equipment such as MRIs and EEGs, biotechnologies such as regenerative tissue growth, and pharmaceutical drugs and biopharmaceuticals.
A JARVIK-7 artificial heart, an example of a biomedical engineering application of mechanical engineering with biocompatible materials for cardiothoracic surgery using an artificial organ.
Subdisciplines within biomedical engineering
Biomedical engineering is a highly interdisciplinary field, influenced by (and overlapping with) various other engineering and medical fields. This often happens with newer disciplines, as they gradually emerge in their own right after evolving from special applications of extant disciplines. Due to this diversity, it is typical for a biomedical engineer to focus on a particular subfield or group of related subfields. There are many different taxonomic breakdowns within BME, as well as varying views about how best to organize them and manage any internal overlap; the main U.S. organization devoted to BME divides the major specialty areas as follows:
- Biomechatronics
- Bioinstrumentation
- Biomaterials
- Biomechanics
- Bionics
- Cellular, Tissue, and Genetic Engineering
- Clinical Engineering
- Medical Imaging
- Orthopaedic Bioengineering
- Rehabilitation engineering
- Systems Physiology
- Bionanotechnology
Sometimes, disciplines within BME are classified by their association(s) with other, more established engineering fields, which can include:
- Chemical engineering - often associated with biochemical, cellular, molecular and tissue engineering, biomaterials, and biotransport.
- Electrical engineering - often associated with bioelectrical and neural engineering, bioinstrumentation, biomedical imaging, and medical devices. This also tends to encompass Optics and Optical engineering - biomedical optics, imaging and related medical devices.
- Mechanical engineering - often associated with biomechanics, biotransport, medical devices, and modeling of biological systems.
Biotechnology and Pharmaceuticals
Biotechnology (see also relatedly bioengineering) can be a somewhat ambiguous term, sometimes loosely used interchangeably with BME in general; however, it more typically denotes specific products which use "biological systems, living organisms, or derivatives thereof." Even some complex "medical devices" (see below) can reasonably be deemed "biotechnology" depending on the degree to which such elements are central to their principle of operation. Biologics/Biopharmaceuticals (e.g., vaccines, stored blood product), genetic engineering, and various agricultural applications are some major classes of biotechnology.
Pharmaceuticals are related to biotechnology in two indirect ways: 1) certain major types (e.g. biologics) fall under both categories, and 2) together they essentially comprise the "non-medical-device" set of BME applications. (The "Device - Bio/Chemical" spectrum is an imperfect dichotomy, but one regulators often use, at least as a starting point.)
Tissue engineering
Tissue Engineering is a major segment of Biotechnology.
One of the goals of tissue engineering is to create artificial organs (via biological material) for patients that need organ transplants. Biomedical engineers are currently researching methods of creating such organs. Researchers have grown solid jawbones and tracheas from human stem cells towards this end. Several bladders actually have been grown in laboratories and transplanted successfully into patients. Bioartificial organs, which use both synthetic and biological components, are also a focus area in research, such as with hepatic assist devices that use liver cells within an artificial bioreactor construct.
Genetic engineering
Genetic engineering, recombinant DNA technology, genetic modification/manipulation (GM) and gene splicing are terms that apply to the direct manipulation of an organism's genes.[1] Genetic engineering is different from traditional breeding, where the organism's genes are manipulated indirectly. Genetic engineering uses the techniques of molecular cloning and transformation to alter the structure and characteristics of genes directly. Genetic engineering techniques have found success in numerous applications. Some examples are in improving crop technology (not a medical application per se; see BioSystems Engineering), the manufacture of synthetic human insulin through the use of modified bacteria, the manufacture of erythropoietin in hamster ovary cells, and the production of new types of experimental mice such as the oncomouse (cancer mouse) for research.
Pharmaceutical engineering
Pharmaceutical Engineering is sometimes regarded as a branch of biomedical engineering, and sometimes a branch of chemical engineering; in practice, it is very much a hybrid sub-discipline (as many BME fields are). Aside from those pharmaceutical products directly incorporating biological agents or materials, even developing chemical drugs is considered to require substantial BME knowledge due to the physiological interactions inherent to such products' usage.
Medical devices
This is an extremely broad category -- essentially covering all health care products that do not achieve their intended results through predominantly chemical (e.g., pharmaceuticals) or biological (e.g., vaccines) means, and do not involve metabolism.
A medical device is intended for use in:
- the diagnosis of disease or other conditions, or
- in the cure, mitigation, treatment, or prevention of disease
Some examples include pacemakers, infusion pumps, the heart-lung machine, dialysis machines, artificial organs, implants, artificial limbs, corrective lenses, cochlear implants, ocular prosthetics, facial prosthetics, somato prosthetics, and dental implants.
Stereolithography is a practical example of medical modeling being used to create physical objects. Beyond modeling organs and the human body, emerging engineering techniques are also currently used in the research and development of new devices for innovative therapies, treatments, patient monitoring, and early diagnosis of complex diseases.
Medical devices are regulated and classified (in the US) as follows (see also Regulation):
- Class I devices present minimal potential for harm to the user and are often simpler in design than Class II or Class III devices. Devices in this category include tongue depressors, bedpans, elastic bandages, examination gloves, and hand-held surgical instruments and other similar types of common equipment.
- Class II devices are subject to special controls in addition to the general controls of Class I devices. Special controls may include special labeling requirements, mandatory performance standards, and postmarket surveillance. Devices in this class are typically non-invasive and include x-ray machines, PACS, powered wheelchairs, infusion pumps, and surgical drapes.
- Class III devices generally require premarket approval (PMA) or premarket notification (510k), a scientific review to ensure the device's safety and effectiveness, in addition to the general controls of Class I. Examples include replacement heart valves, hip and knee joint implants, silicone gel-filled breast implants, implanted cerebellar stimulators, implantable pacemaker pulse generators and endosseous (intra-bone) implants.
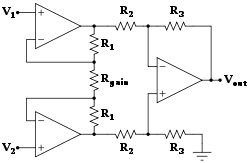
Biomedical instrumentation amplifier schematic used in monitoring low voltage biological signals, an example of a biomedical engineering application of electronic engineering to electrophysiology
Medical imaging
Medical/Biomedical Imaging is a major segment of Medical Devices. This area deals with enabling clinicians to directly or indirectly "view" things not visible in plain sight (such as due to their size, and/or location). This can involve utilizing ultrasound, magnetism, UV, other radiology, and other means.
Imaging technologies are often essential to medical diagnosis, and are typically the most complex equipment found in a hospital including:
- Fluoroscopy
- Magnetic resonance imaging (MRI)
- Nuclear Medicine
- Positron Emission Tomography (PET) PET scansPET-CT scans
- Projection Radiography such as X-rays and CT scans
- Tomography
- Ultrasound
- Optical Microscopy
- Electron Microscopy
Implants
An implant is a kind of medical device made to replace and act as a missing biological structure (as compared with a transplant, which indicates transplanted biomedical tissue). The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone or apatite depending on what is the most functional. In some cases implants contain electronics e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents.
A prosthetic eye, an example of a biomedical engineering application of mechanical engineering and biocompatible materials to ophthalmology
Clinical engineering
Clinical engineering is the branch of biomedical engineering dealing with the actual implementation of medical equipment and technologies in hospitals or other clinical settings. Major roles of clinical engineers include training and supervising biomedical equipment technicians (BMETs), selecting technological products/services and logistically managing their implementation, working with governmental regulators on inspections/audits, and serving as technological consultants for other hospital staff (e.g. physicians, administrators, I.T., etc.). Clinical engineers also advise and collaborate with medical device producers regarding prospective design improvements based on clinical experiences, as well as monitor the progression of the state-of-the-art so as to redirect procurement patterns accordingly.
Their inherent focus on practical implementation of technology has tended to keep them oriented more towards incremental-level redesigns and reconfigurations, as opposed to revolutionary research & development or ideas that would be many years from clinical adoption; however, there is a growing effort to expand this time-horizon over which clinical engineers can influence the trajectory of biomedical innovation. In their various roles, they form a "bridge" between the primary designers and the end-users, by combining the perspectives of being both 1) close to the point-of-use, while 2) trained in product and process engineering. Clinical Engineering departments will sometimes hire not just biomedical engineers, but also industrial/systems engineers to help address operations research/optimization, human factors, cost analysis, etc. Also see safety engineering for a discussion of the procedures used to design safe systems.






No comments:
Post a Comment